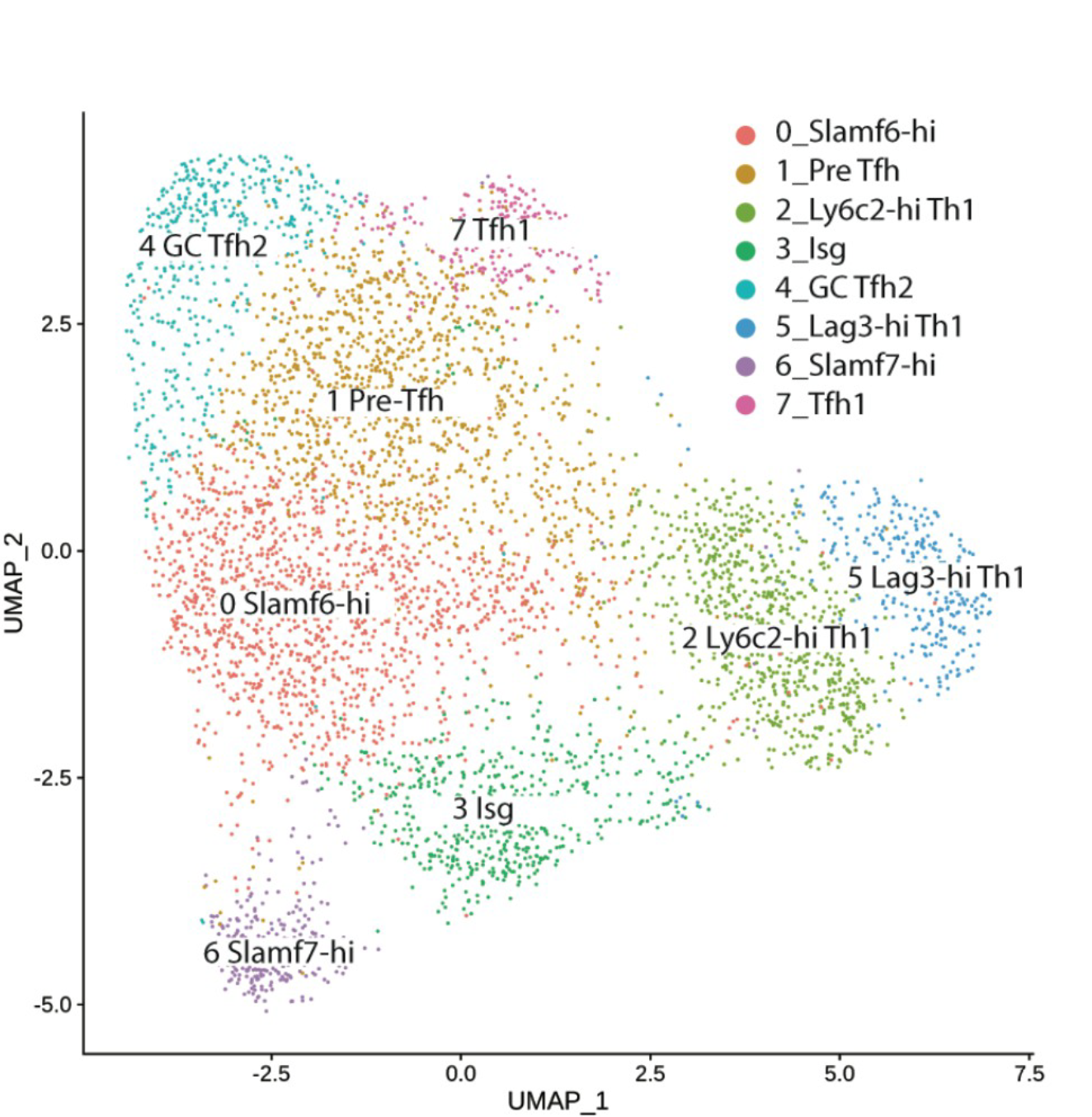
CD4+ T cell differentiation during chronic infection and cancer
CD4+ T cells play a critical role in sustaining CD8+ T cell responses and humoral immunity during chronic viral infection. CD4+ T cells also contribute to protective anti-tumor immunity during several different types of cancer. Nevertheless, how persistent exposure to antigen and inflammation shapes helper T cell differentiation remains incompletely understood. Our lab is interested in determining how prolonged antigen stimulation impacts the differentiation and helper function of virus- and tumor-specific CD4+ T cells. We additionally seek to dissect the cellular and molecular mechanisms by which CD4+ T cells interact with other immune cell subsets, such as DCs, B cells, and CD8+ T cells to facilitate protective immunity, as well as how to harness CD4+ T cell “help” therapeutically to improve control over chronic infection and cancer.
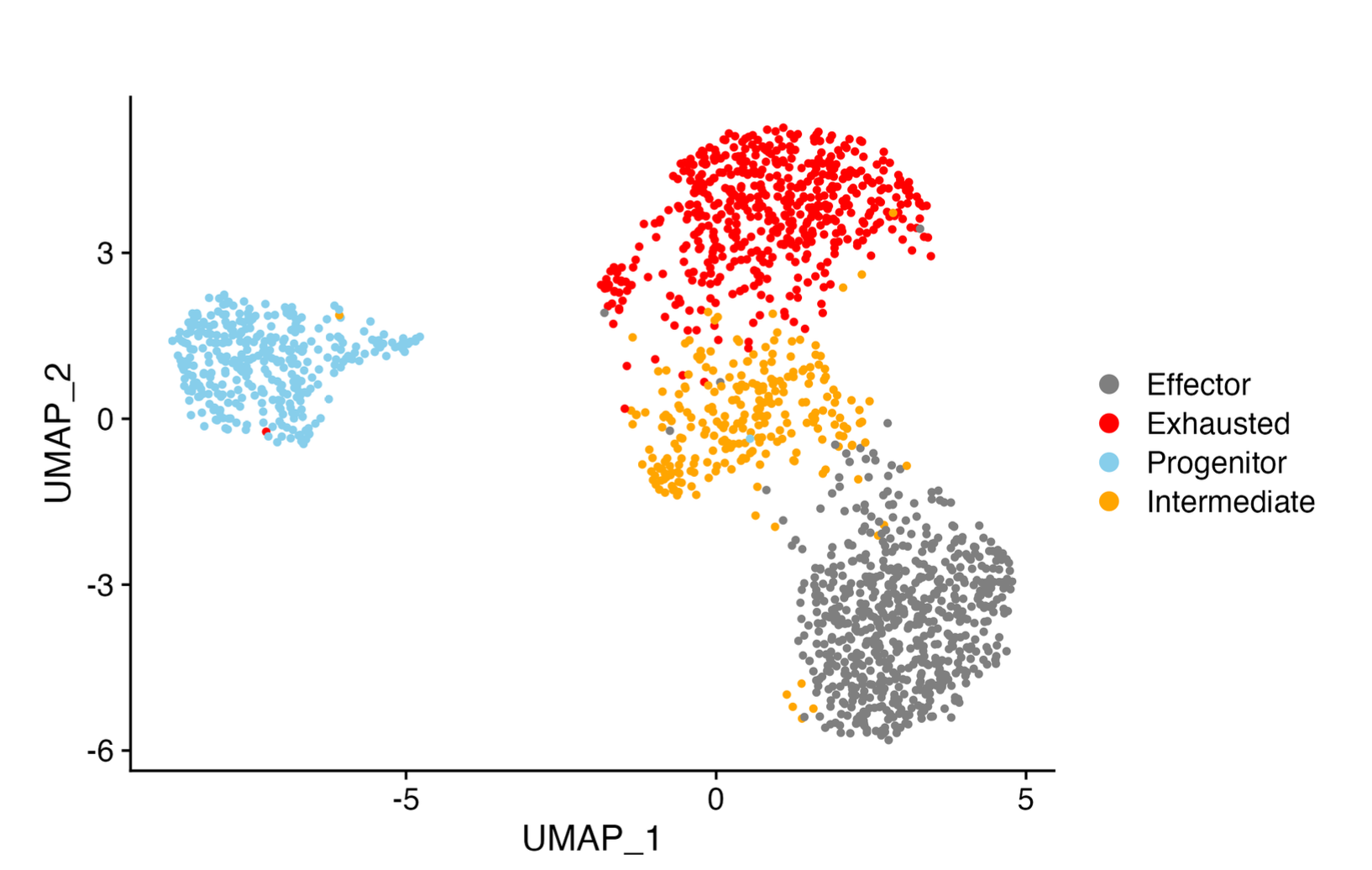
T cell “exhaustion”
In response to persistent antigen exposure, such as during chronic viral infection or cancer, CD8+ T cells often display a progressive loss of effector function and diminished proliferative potential. This deterioration in function, coined T cell “exhaustion” is further accompanied by the upregulation of multiple coinhibitory receptors and eventual clonal deletion. Although previously considered to be a homogenous population of dysfunctional T cells, emerging evidence indicates that the “pool” of exhausted T cells is comprised of multiple heterogenous populations, including a self-renewing progenitor subset that transitions through an intermediate state before bifurcating into either terminally exhausted cells or highly functional effector cells that are critical for viral control. Our lab is interested in determining the molecular factors underpinning this multifaceted differentiation process in the hopes of identifying novel regulators of T cell exhaustion that can be therapeutically targeted to improve control over chronic infection and cancer.